Dr. Karen Keough, Board-Certified Child Neurologist & Epileptologist and Chief Medical Officer, Compassionate Cultivation
Every practicing neurologist faces questions from their patients about medical cannabis. Since February 2018, when the first prescriptions were filled under the Texas Compassionate Use Act, we have learned much about its potential clinical benefits and how best to integrate it into patient care. As an early adopter of medical cannabis therapy for my patients with refractory epilepsy, and now for patients with autism and spasticity, I’d like to share some of that experience in hopes that more patients will gain access to this important therapeutic option.
In this review, I will provide some basics about medical cannabis, share salient published evidence for its efficacy and safety, and summarize my own experience with 135 patients who have undergone cannabis therapy. I will then touch on the expansion the Texas Compassionate Use Act in June 2019 that now includes several new conditions, most of them relevant primarily to neurologists.
Medical Cannabis and its Two Key Compounds, CBD and THC
The human endocannabinoid system (ECS) was discovered in the 1990s during research on the effects of cannabis on the human body. Two cannabinoid receptors (CB1 and CB2) have been discovered. Both moderate a wide range of systems including memory, mood, motor function and pain perception and immune function. CB1 receptors are found mainly in the brain and central nervous system; CB2 receptors, in the immune system. Cannabis plants produce a thick substance containing over 100 different cannabinoid compounds. The most common cannabinoid compounds are CBD (cannabidiol) and THC (tetrahydrocannabinol). Hemp is a subtype of cannabis that contains low concentrations of THC, a distinction relevant to laws related to agriculture and commercial sales of cannabis products.
A Brief History of Cannabis as Medicine
Used medically for centuries, cannabis was first described in the United States Pharmacopoeia in 1850. Its use and sale were federally restricted for the first time in 1937 under the Marihuana Tax Act. In 1942, it was removed from the United States Pharmacopoeia. The passage of various laws in the 1950s and the Controlled Substances Act of 1970 effectively criminalized cannabis use and possession and limited research on it.
In 1996, through its Compassionate Use Act, California became the first state to sanction access to and use of botanical cannabis for medicinal purposes with authorization by a physician. By January 2019, 36 states, the District of Columbia, Guam, and Puerto Rico had enacted legislation governing medicinal cannabis sale and distribution; 21 states and the District of Columbia had decriminalized marijuana possession in small amounts, and nine states (Alaska, California, Colorado, Maine, Massachusetts, Michigan, Nevada, Oregon, and Washington) and the District of Columbia had legalized adult recreational use of marijuana. Each state can define its own medical cannabis program, so definitions vary widely. Texas defines medical cannabis as "low-THC" cannabis derived from Cannabis sativa or any derivative that contains no more than 0.5% tetrahydrocannabinol (THC).
The Texas Compassionate Use Act
Originally passed in June 2015, the Texas Compassionate Use Act allowed the creation of a system for physician prescription of high-CBD, low-THC medical cannabis exclusively for the treatment of refractory epilepsy. In February 2018, the first Texas dispensary (Compassionate Cultivation) opened in Manchaca, just south of Austin. One of only three licensed dispensaries in the state at the time, it officially began producing, processing, and distributing the only authorized formulation at the time: a 20:1 ratio of CBD-to-THC in compliance with the state’s maximal concentration of THC (0.5%) and minimal concentration of CBD (10%). This formulation became known as Lonestar CBD.
Experience with 20:1 traditional Lonestar CBD
The choice of refractory epilepsy as the first target for medical cannabis therapy in Texas was prescient. Between 2017and 2018, three landmark publications confirmed the safety and efficacy of high CBD therapy for prevention of seizures in patients with complicated refractory epilepsy. 1,2,3 These studies supported the long-held expectations of many patients that medical cannabis in the form of cannabidiol could provide benefit in the most challenging cases. These studies also identified potential side effects and gave important insight into relevant dosages. Although generalization of these data to artisanal CBD therapy is fraught with unwarranted assumptions, these early studies provided a foundation for prescribing empiric CBD/THC therapy to patients with refractory, life-threatening seizures. Meanwhile, the establishment of highly regulated state-sanctioned CBD dispensaries provided a safe, reliable source of therapeutic CBD. Subsequently, my partners and I are conducting an IRB-approved observational study of CBD treatment in 135 patients with complex refractory epilepsy from February 2018 to January 2020. (see Figure 1).
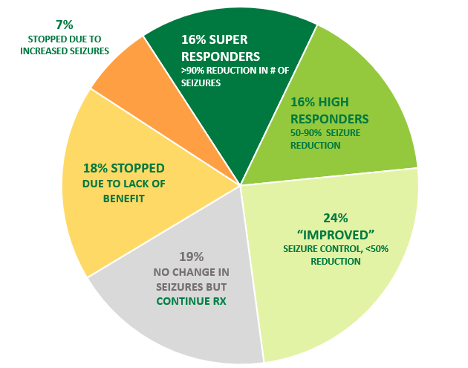
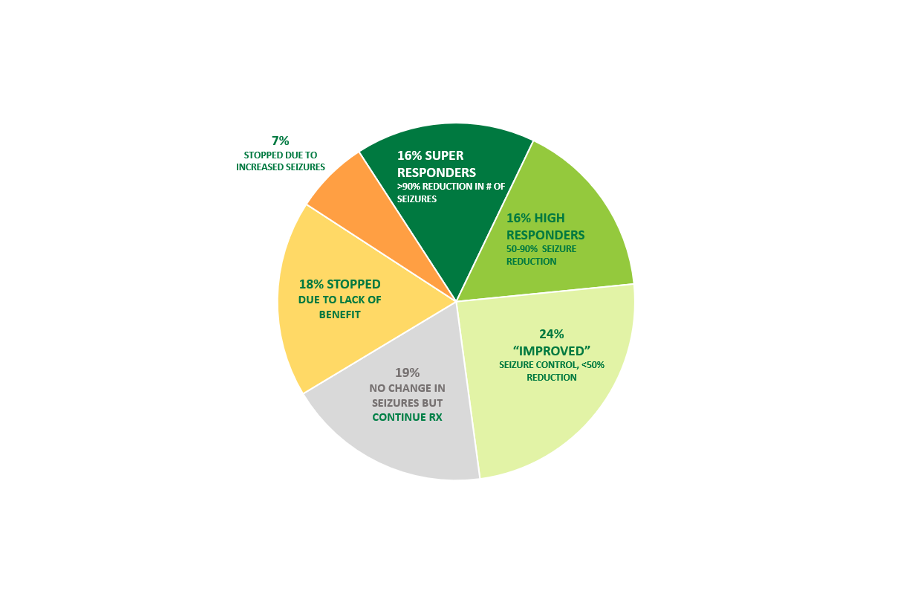
Figure 1: 135 patients treated with Lonestar CBD 20:1 formulation with or without Epidiolex February 2018 – January 2020. Duration of follow-up ranged from 1 month – 22 months.
Here are the most important lessons I have learned:
- For some patients, CBD is the best thing they have ever tried for epilepsy.
Four of my patients (3%) on empiric monotherapy with CBD or CBD/THC are now seizure-free. All four had previously failed to achieve seizure control on 3-5 trials of other seizure medications. These cases of children with severely refractory epilepsy now fully controlled by the empiric use of one medication highlight the importance of a trial of CBD therapy in highly refractory patients.
- Seizure-freedom is not the sole measure of success.
Because the original Compassionate Use Program in Texas was restricted to patients with refractory epilepsy, every patient in our trial had failed at least 2 prior trials of seizure medication, and often several more. Kwan and Brodie’s landmark study famously demonstrated the challenge of achieving full seizure control in a refractory population.4 Decreasing seizure frequency and severity with minimal side effects are realistic and vital goals of treatment. In my series, 32% of patients achieved >50% seizure reduction during a follow-up period of 1-22 months (>6 months in 78 patients). An additional 24% reported a worthwhile reduction.
- CBD therapy may exacerbate seizures. This was observed in the Epidiolex trials as well. Although relatively infrequent (7% in my series), I verified this outcome by a second trial in several of these patients. A few other patients responded well at low doses, had more seizures at higher doses, and then improved when the CBD dose was decreased.
- Most of my patients (75%) retain CBD as part of their treatment regimen.
For most patients, the combination of improved seizure control and a beneficial profile warrant continued use of CBD. CBD not only has few adverse effects, but many parents and patients report improved alertness, responsiveness, and expressive language. These reports are difficult to quantify and may in part represent placebo effects. However, unlike most of my follow-up conversations with patient and families about other medical therapies, conversations about CBD usually focus on “what else is better”, instead of what is “new and worse”. One powerful demonstration of CBD’s beneficial impact is that many patients continue using it, despite the considerable out-of- pocket cost of dispensary CBD/THC to their families.
- THC contributes independent benefits compared to CBD alone. Because the Texas Compassionate Use Act allowed for initiation of therapy in February 2018, and Epidiolex was not widely available until a year later, 75 patients in this series had long-term therapy with dispensary CBD before switching to Epidiolex therapy. Figure 2 shows the response of patients switched from dispensary Lonestar CBD to Epidiolex. For some patients, the change was unremarkable. In other patients, the ability to use higher doses (because insurance covered the cost of Epidiolex) resulted in improved seizure control. However, in 43% of patients, seizures increased in those who had achieved better control on dispensary CBD. In another 17% of cases, new side effects appeared that had not been seen before switching to Epidiolex. In both of these groups, the reinstitution of THC therapy in combination with Epidiolex alleviated these problems. I now treat many patients with a combination of low-CBD (balanced formulation) Lonestar CBD with Epidiolex. I now transition this way proactively by converting high-CBD treatment to balanced formulation when I add Epidiolex. To better understand this combination, please read the next portion of the article regarding the expansion of the Texas Compassionate Use Act that resulted in new formulation options in Texas. Prescribing THC to children understandably raises concerns about adverse effects. According to data that come mainly from studies of recreational drug use or animal models, learning, memory, and attention may be impaired after recent cannabis use (within 24 hours) and even after cannabis use is discontinued. Cannabis abuse may increase the risk of schizophrenia and other psychoses, and use of THC at higher concentrations has been associated with greater risk in population studies.5 Although these risks are related to dose, what a “safe” dose is remains unknown. Moreover, differential risk in developing brains has not been extensively studied. Therefore, I discuss these concerns with all my patients and their parents before initiating CBD therapy. How the short- and long-term risks of CBD/THC therapy compare with the risks of treatment with standard prescription medication are also unknown. However, given the serious symptoms and diagnoses that inspire these families to explore CBD/THC treatment in the first place, most patients and caregivers are willing to accept these risks.
Expansion of the Texas Compassionate Use Program (T-CUP).
In June 2019, based on the success of the intractable epilepsy program, the T-CUP was expanded. It further expanded in June 2021, allowing medical cannabis treatment for a broader set of qualifying conditions, including:
- all forms of epilepsy and other seizure disorders
- autism
- multiple sclerosis
- spasticity
- amyotrophic lateral sclerosis
- cancer
- post-traumatic stress disorder (ptsd)
- other neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, and many more as defined in a list from the Texas Department of Health and Human Services.
Discussion of approach to treatment for these various conditions is beyond the scope of this article, though I will expand on treatment for autism below. For more extensive references on medical cannabis in the treatment of these conditions, Compassionate Cultivation has compiled a complete repository of condition-specific medical studies on their website (www.texasoriginal.com).
Other important changes in the T-CUP included:
1. removing the requirement for a second qualified medical opinion,
2. eliminating the 10% ‘floor’ for CBD content in products. The latter change allowed T-CUP dispensaries to offer a variety of THC:CBD ratios that target other symptoms that benefit from lower CBD:THC ratios--symptoms such as pain, muscle spasms/spasticity, lack of appetite, insomnia, restlessness and others.
Understanding Ratios and Formulations
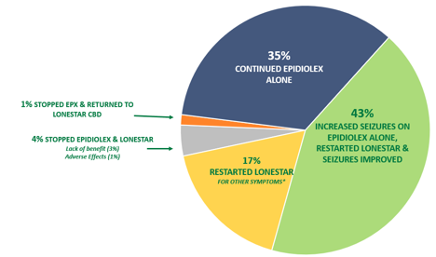
Medical cannabis can produced in various formulations with differing ratios of CBD and THC. However, the maximum concentration of THC remains limited to 0.5% THC by weight. A useful clinical distinction is comparing high-CBD formulations with balanced (low-CBD) formulations. All formulations of Lonestar CBD have the same state-mandated maximum THC concentration of 0.5%. High-CBD formulations contain up to 20 times the concentration of CBD compared to THC. This includes the original version of Lonestar CBD with a 20:1 ratio: 100 mg/mL CBD + 5 mg/mL THC. Clinical research suggests that in addition to providing a positive impact on seizure control, high-CBD formulations can alleviate some symptoms associated with autism such as anxiety, depression, restlessness and disruptive behavior.
Balanced formulations have similar levels of CBD and THC by weight. Lonestar CBD is also available in 3:1 ratio (15 mg/mL CBD + 5 mg/mL THC) and 1:1 ratio (5 mg/mL CBD + 5 mg/mL THC). Clinical research suggests that balanced formulations can have a positive impact on symptoms associated with T-CUP qualifying conditions such as terminal cancer, autism and other neurodegenerative disorders, as illustrated in the chart in Figure 3. Some patients may experience multiple symptoms that could respond to either high-CBD formulations or balanced formulations, or a combination of both. I utilize the 1:1 Lonestar formulation frequently in combination with Epidiolex, which has no measurable THC content, to enhance seizure control and to mitigate other symptoms that may be present prior to treatment or that may be provoked by high-CBD therapy, such as insomnia, anorexia, and agitation.
How Does a Doctor Register to Prescribe Medical Cannabis in Texas?
Joining the Compassionate Use Registry is simple: visit the Texas Department of Public Safety Compassionate Use Registry portal at https://curt.dps.texas.gov/app/application/physicianEmailInvitation.xhtml.
Enter your name and email address – an email including a link to the Physician Registration Wizard will be sent to you. To register, you will need 1.) the Texas Medical Board License number, 2.) your American Board of Medical Specialties number and 3.) your Texas Driver’s License audit number. After completing the application, you will receive a confirmation email and tracking number. Note that verification by the Department of Public Safety can take between 1 – 10 business days. Originally, all physicians who registered were listed on the CURT website, but now listing is optional. Physicians must expressly provide consent in order to be listed.
How Should a Physician Approach Dosing?
Like most new medications, dosage recommendations for cannabis medicines are best established according to existing scientific research, careful integration of high-quality anecdotal evidence, and repeated evaluation of individual patient outcomes. The process of finding the correct dose differs for each patient, determined through an empiric titration process. The dosing strategy of cannabidiol starts at 5 mg/kg/day, targeting 10 mg/kg/day and ranging up to 20 mg/kg/day or higher. These doses are not sustainable financially for most adult patients, though smaller children can reach high per-kilogram dosing at affordable rates. In my series of epilepsy patients utilizing 20:1 Lonestar CBD, many patients achieved improvement in seizure control at much lower doses than suggested in the Epidiolex trials. I start with a dose of 0.5 mg/kg/day (minimum dose 5 mg = 0.05 mL) and titrate weekly. Most patients did well on 3-8 mg/kg/day. Some went higher than 10 mg/kg/day and this can achieve better results in some patients, but cost can be limiting. The ability to achieve higher CBD dosing through insurance approval of Epidiolex is game-changing for some of my patients. I always seek Epidiolex approval in patients who have demonstrated a response to CBD therapy. This has become increasingly successful as Epidiolex has had more time on the market and insurers recognize that some patients benefit remarkably well in off-label use.
Dosing balanced formulations: THC is far more potent for producing a clinical effect per milligram compared to CBD. Whereas patients utilizing high CBD treatments will take doses in the hundreds of milligrams per day, THC can have clinical impact with only a few milligrams per day. CBD is included with THC in the balanced formulation intending to mitigate some of the concerning effects of THC, including euphoric effect, and risk of psychosis. The ideal combination of CBD and THC is not well understood and is likely to vary depending on the targeted symptoms and/or individual patient responses. Dosing balanced formulations is best conceptualized as primarily dependent on the THC component. Variation of 1 or 2 milligrams/day can have a differential impact. The amount of THC that may impart a euphoric effect is not clearly determined but may be as low as 5 mg without CBD in the mix. Doses above 10 mg at a time or above 20 mg/day should be considered with caution. Clinical studies show the starting dose of balanced formulations for most targeted conditions/symptoms will range from 2.5-5mg of THC per day, and lower in smaller patients. This guidance is especially important when initiating therapy for patients with no prior experience using cannabis medicine. Medically sensitive and complex patients, such as young children or those taking multiple concomitant seizure medications, should be started at a lower dose and titrate more slowly. Less sensitive patients can be started at a somewhat higher initial dosage.
For optimal delivery of medication, it should be placed inside the mouth between the cheek and gums or on the tongue and allowed to be absorbed. Compassionate Cultivation offers a spray formulation of the 20:1 formulation that optimizes mucosal absorption. The medication can be swallowed rather than absorbed in the mouth, but less medication will reach circulation due to first-pass hepatic metabolism.
AUTISM
The expansion of eligible conditions by Texas House Bill 3703 in June 2019 added autism to the list of eligible conditions for the Texas Compassionate Use Program. Studies examining CBD/THC therapy for autism are much smaller than studies of epilepsy patients, and they are observational without placebo controls. Several larger scale phase III randomized placebo-controlled trials are underway internationally, but those results are still unknown. Poleg et al published a useful review or articles addressing CBD therapy for autism in 2019.6
True to its name as a spectrum disorder, autism can manifest with mild to severe behavioral symptoms that often prove difficult to manage pharmacologically. These challenges motivate patients and caregivers to seek alternatives that might improve efficacy, mitigate side effects, or both. Published studies of patients with autism suggest benefits from CBD in alleviating anxiety, attention, hyperactivity, impulsivity.7 THC has more beneficial impacts on insomnia and aggressive behavior. In my experience, the essential role of THC in managing these challenging symptoms frequently manifests itself when patients convert from Lonestar CBD to Epidiolex, which does not contain any THC but only CBD. These are the two most common symptoms other than increase of seizures that have led me to restart or maintain THC as part of the treatment regimen when patients transition to Epidiolex. Because of the apparently complementary effects of CBD and THC, they are “good partners” in therapy. Finding the right ratio of CBD and THC in combination for individual symptoms, conditions and patients is an empiric process, since studies assessing various ratios are not available for guidance. The evidence-base to guide CBD/THC treatment of autism will expand considerably soon with the expected conclusion and publication of several larger, placebo-controlled trials underway involving CBD and CBDV (another cannabinoid not yet in wide clinical use) as mentioned in the Poleg article.
TERPENES AND “WHOLE PLANT” FORMULATIONS
Terpenes are chemicals found in plants that create aromas or flavors. Any given terpene can be found to exist in a variety of different plants. For instance, α-Pinene is found in pine needles. Linalool is found naturally in lavender. ß-Caryophyllene is found in black pepper, cloves, rosemary and hops. Limonene is common in citrus fruit. Many terpenes exist in cannabis, and the mixture depends on the individual plant. Because the plants grown at the dispensary are clones, their terpene profile is uniform. There is much speculation about the role of terpenes as a contributor to the therapeutic effects of medical cannabis treatment, referred to as the “entourage effect”. Although health implications have been attributed individually to many terpenes, the studies supporting these association generally were derived from animal models8, and no human studies have carefully examined the entourage effect. Many patients express interest in CBD/THC preparations that include terpenes. At Compassionate Cultivation, the “Plus” formulation of each product contains the standard mixture of terpenes that is derived from their stock plant and is a consistent additive between batches. I have a few patients who utilize the Plus form at their request. I have not had any patients display a clear difference with the addition of terpenes, but only a few of my patients have requested it. Other artisanal CBD suppliers offer “whole plant” formulations that include both terpenes and also a mixture of other cannabinoid compounds that are extracted along with CBD and THC when the plants are processed. This complex mixture is highly variable when sourced from producers who have varied cannabis sources. Several cannabinoids have become the focus of early studies seeking additional clinical applications for medical cannabis. As a prescriber, I prefer a pure preparation without terpenes or other cannabinoids, so that I have a full understanding of what the patient is taking and I can guide therapy within the context of established evidence regarding the effects of CBD and THC. Other prescribers have shared with me that their review of evidence on the impact of terpenes and other cannabinoids supports some expectation of additional benefits. I encourage prescribers to become informed about these issues, because patients often have read a lot about the topic and will ask for opinions.
Where Can I Learn More About Prescribing Medical Cannabis in Texas?
Compassionate Cultivation has compiled an excellent repository of research on medical cannabis as it relates to the conditions approved by the Texas legislature can be found at the Compassionate Cultivation website: https://texasoriginalcc.com/prescribing-medical-marijuana/. For more information, contact the dispensary at (512) 614-0343.
Dr. Karen Keough is a board-certified pediatric neurologist who specializes in treating intractable epilepsy at Child Neurology Consultants of Austin. She serves as Chief Medical Officer of Compassionate Cultivation. She initiated the child neurology residency program at Dell Medical School at the University of Texas at Austin and continues to serve as faculty.
References:
This article was originally published in the Texas Neurological Society's Summer 2020 Broca's Area Newsletter.
1. Devinsky et al, Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrom N Engl J Med 2017; 376:2011-2020
2. Thiele et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018 Mar 17;391(10125):1085-1096.
3. Devinsky et al, Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N Engl J Med 2018; 378:1888-1897
4. Kwan P, Brodie MJ. (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319.
5. The Health Effects of Cannabis and Cannabinoids: the current state of evidence and recommendations for research. National Academies of Science, Engineering and Medicine, 2017. Nationalacademies.org/CannabisHealthEffects.
6. Poleg et al. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Progress in Neuropsychopharmacology & Biologic Psychiatry 89 (2019) 90-96
7. Schleider et al. Real Life Experience of Medical Cannabis Treatment in Autism: Analysis of Safety and Efficacy. Scientific Reports 2019 9:200.
8. Koziol A et al. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini-Reviews in Medicinal Chemistry 2014, 14, 1156-1168